Safety and Effectiveness of Posterior Cervical Stabilization System (PCSS) as an adjunct to posterior cervical fusion (PCF), when used in combination with ACDF in the treatment of multi-level cervical degenerative disease. (“FUSE Study”)

Do you have pain in your neck, shoulders, arms, or hands?
Does your pain interfere with:
- Working?
- Reading?
- Driving?
- Sleeping?
- Lifting heavy objects?
- Self-care (washing, dressing, etc.)?
If so, ask your healthcare provider if you qualify to participate in the clinical research “FUSE” Study involving the Posterior Cervical Stabilization System (PCSS™).
What are the goals of the FUSE Clinical Study?
This study is designed for patients that need to have 3-levels fused in their neck.
This study will evaluate the safety & effectiveness of PCSS and whether it improves patients' fusion rates when used as part of Circumferential Cervical Fusion.
What should I expect from participating in the FUSE Study?
Prior to the study, participants will visit their doctor for a one-time assessment before being randomly assigned to receive either Circumferential Cervical Fusion (CCF) or ACDF.
Participants will return to you your doctor’s office six times after surgery, after 6 weeks and 3, 6, 9, 12, and 24 months to track your symptom improvement and overall surgical outcome.
At 12 and 24 months after surgery, participants will receive x-rays and CT scans (also known as “CAT Scans”) to confirm successful fusion of your treated vertebrae (spinal levels). These assessments are paid for by the study with no cost to participants.
Participants will discuss the potential risks and benefits with their doctor.
Cervical Degenerative Disc Disease (DDD)
DDD is the breakdown of an intervertebral disc in the spine. DDD can result in a loss of space within the foramen, an opening through which nerve roots exit the spine.1
This can pinch, irritate, or compress nerve roots and lead to chronic and debilitating pain, numbness, and/or burning sensations in the neck, shoulders, and/or arms.
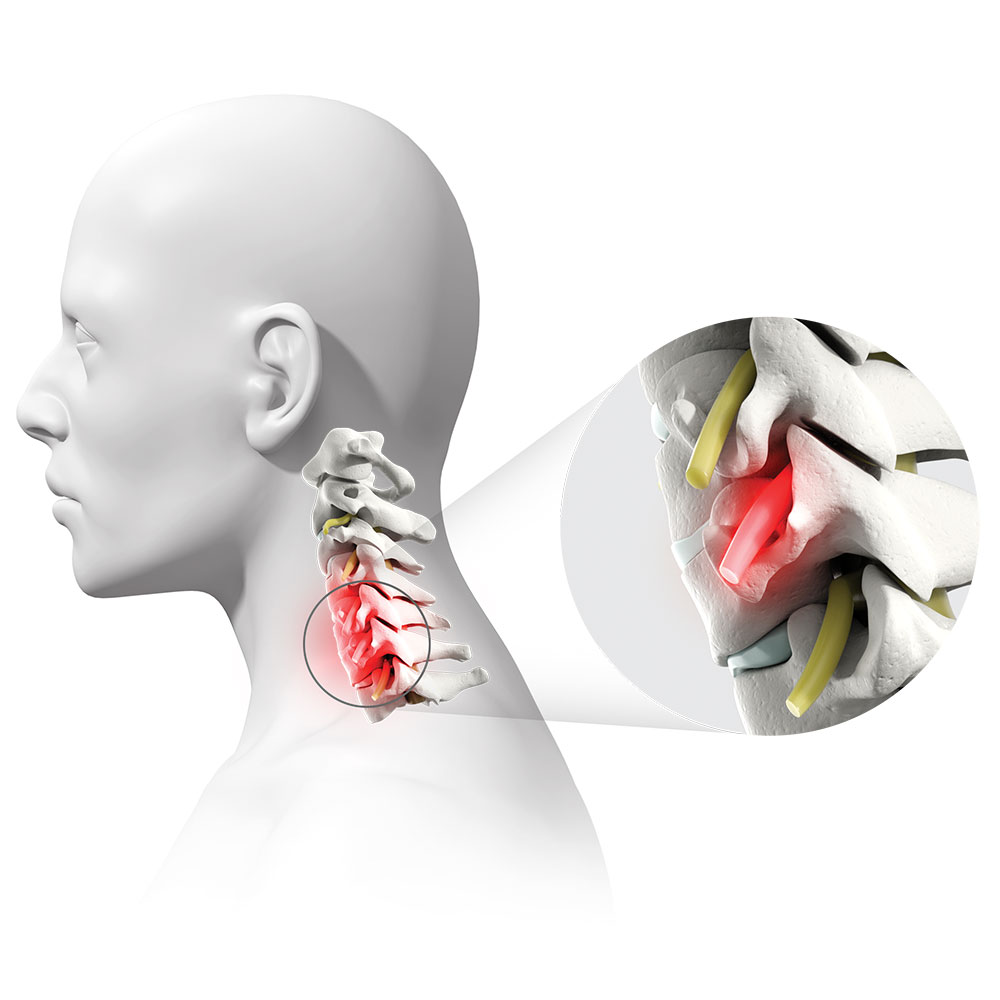
Pictured: A nerve root that has been pinched, irritated, or compressed.
What is Anterior Cervical Decompression & Fusion (“ACDF”) & how does it work?
ACDF is a common surgery for patients with cervical DDD where a patient’s disc in their spine is removed and replaced with a spacer or implant made of metal, plastic, or bone. In the FUSE Study, the surgeon will use bone to replace the disc and a metal plate to stabilize the spine.
A multilevel ACDF is when more than one spinal level is treated. While effective, multilevel ACDFs are associated with significantly higher rates of nonunion, which occurs when the bones of the spine fail to heal properly.2–4
This risk of nonunion increases with the number of levels treated.2,3
A nonunion may require another surgery in order to resolve symptoms.
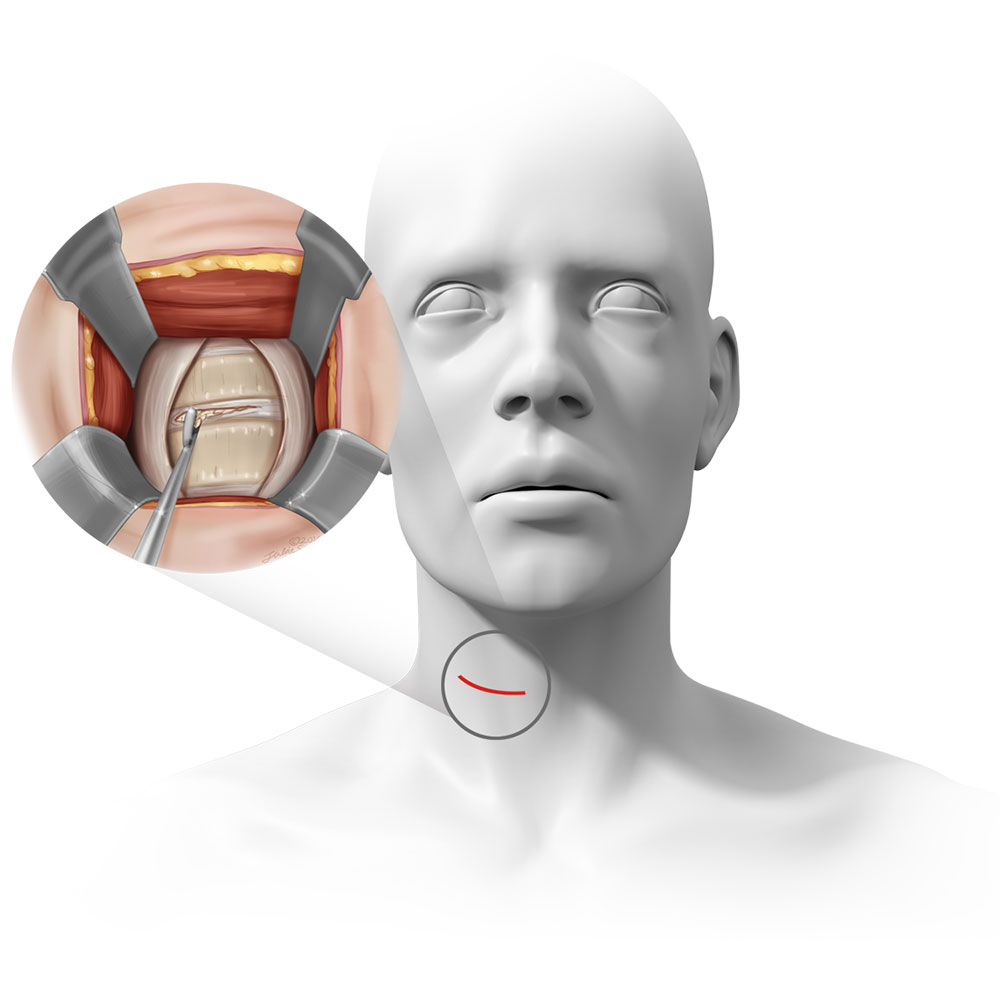
Pictured: ACDF location, incision, and surgical exposure.
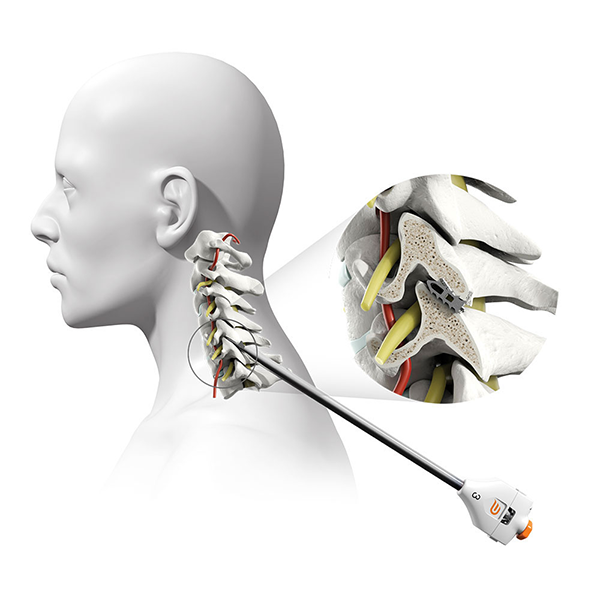
What is PCSS & how does it work?
The Posterior Cervical Stabilization System (“PCSS Device”) is a small titanium implant that is surgically placed in the facet joints on the back side of the neck. PCSS, as part of posterior cervical fusion (PCF, see image), is designed to be used with an ACDF to immobilize and stabilize the spine, which may improve fusion rates.
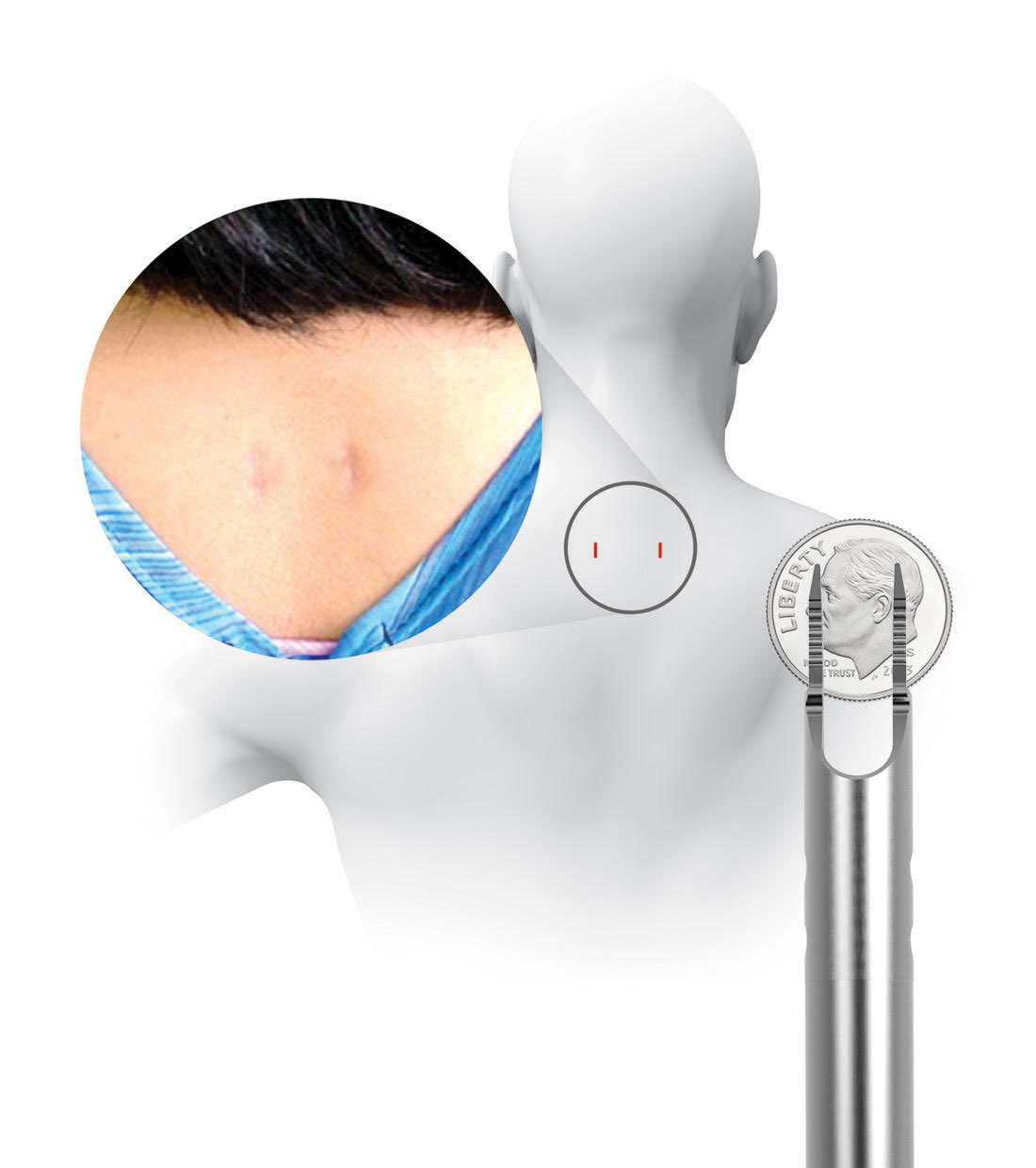
Prior to placing the implants into the facet joints, the doctor will prepare the target joints with PMT surgical instruments and then deliver PCSS through a small tube to minimize muscle and tissue damage. The PMT surgical instruments are commercially available and indicated for posterior cervical fusion in patients with degenerative disc disease.
What is a Circumferential Cervical Fusion (“CCF”)?
A Circumferential Cervical Fusion is when an anterior fusion (ACDF) is combined with a posterior cervical fusion (PCF).
Circumferential Cervical Fusion is indicated for complex patients, provides greater stability, and offers a higher rate of fusion than anterior fusion alone.* 5–8
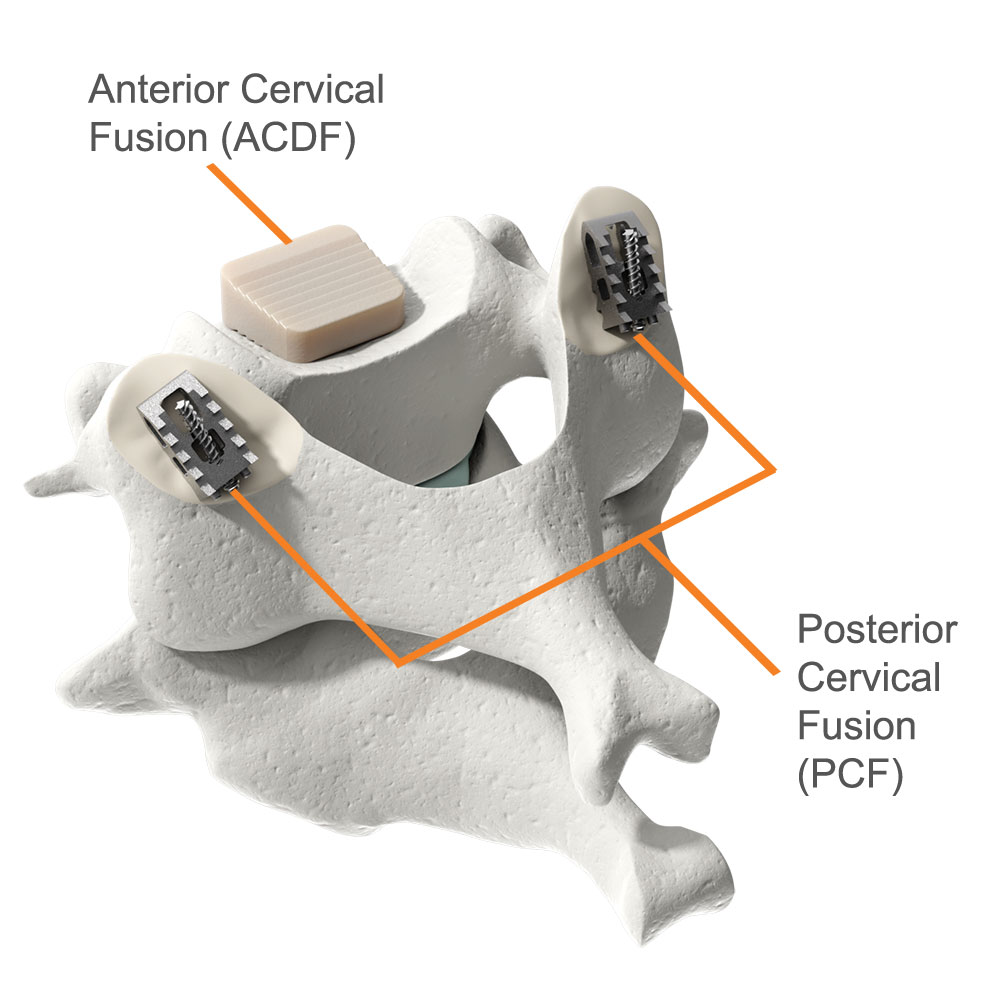
Pictured: implants used in the ACDF and PCF procedures.
Video: Implants for Anterior, Posterior, & Circumferential Fusion
What clinical information is already available for PCSS?
PCSS is an investigational device. Providence Medical Technology, the company that designed PCSS, conducted a clinical study in the Philippines using a similar device that was also placed into the facet joints.
This study demonstrated: 9,10
- A decrease in pain
- Lower Neck Disability Index (NDI) scores — a quality of life scale
- High fusion rate
In addition, surgeons in the U.S. have used the PCSS and similar devices in their patients with favorable outcomes and a low reported device-related complication rate of 3.4%.11
In a biomechanical study, the combination of ACDF (anterior fusion) and a PCF (posterior fusion) using PCSS significantly increased spinal stability versus ACDF alone.12
Prior to deciding whether to participate in the study, please discuss the risks and benefits of PCSS and the study with your doctor.
Enrollment in the FUSE Study is complete.
The study no longer accepting subjects.
For detailed information about this cervical fusion clinical study visit: clinicaltrials.gov
CAUTION — PCSS is an investigational device. Limited by federal law to investigational use.
Official Study Name
Safety and Effectiveness of Posterior Cervical Stabilization System (PCSS) as an adjunct to posterior cervical fusion (PCF), when used in combination with ACDF in the treatment of multi-level cervical degenerative disease. (“FUSE Study”)

About Providence Medical Technology
Our Purpose is to improve clinical outcomes for high-risk patients and prevent surgical failures of the cervical spine.
Our Mission is to establish Circumferential Cervical Fusion (CCF) as the standard of care for high-risk patients.
3875 Hopyard Rd. Suite 300
Pleasanton, CA 94588
References
1. Lu J, et al. (2000) Clin Orthop Relat Res.
2. Yu BS, et al. (2017) Nerve.
3. Shen HX, et al. (2010) Spine (Phila Pa 1976).
4. Zigler JE, et al. (2016) Spine (Phila Pa 1976).
5. Song KJ, et al. (2010) J. Bone Joint Surg. Br.
6. Dmitriev AE, et al. (2007) Spine (Phila Pa 1976).
7. Mummaneni PV, et al. (2007) Neurosurgery.
8. Harel R, et al. (2013) J Spinal Disord Tech.
9. McCormack B, et al. (2013) J Neurosurg Spine.
10. Siemionow K, et al. (2016) J Neurol Surg A Cent Eur Neurosurg.
11. Siemionow K, et al. (2017) J Craniovertebr Junction Spine.
12. Voronov LI, et al. (2016) Medical Devices (Auckl).
* Providence products were not used in these studies.
Copyright © 2020, Providence Medical Technology. All rights reserved.