
Have you been told you need fusion surgery for your neck?
You may be eligible to participate in a clinical study.
This clinical study is evaluating whether the Posterior Cervical Stabilization System (“PCSS”) improves fusion rates for cervical fusion patients when used as part of Circumferential Cervical Fusion.
Study Hypothesis: Patients who receive circumferential fusion with PCSS will have a higher rate of fusion and fewer surgical failures.
Am I Eligible?
Call (833) 321-3873
for more information
Innovative, Investigational Treatment Technology
The Posterior Cervical Stabilization System (“PCSS Device”) is a small titanium implant that is surgically placed in the facet joints on the back side of the neck. PCSS, as part of posterior cervical fusion (PCF, see image), is designed to be used with an ACDF to immobilize and stabilize the spine, which may improve fusion rates.
Prior to placing the implants into the facet joints, the doctor will prepare the target joints with PMT surgical instruments and then deliver PCSS through a small tube to minimize muscle and tissue damage. The PMT surgical instruments are commercially available and indicated for posterior cervical fusion in patients with degenerative disc disease.
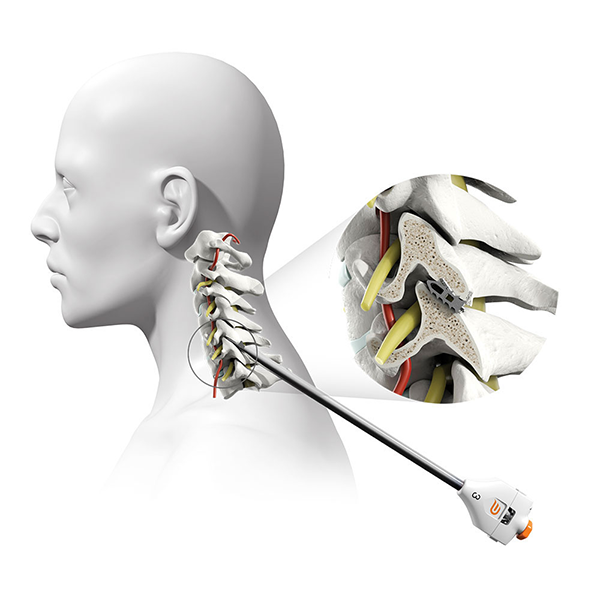
Pictured: PCSS in a facet joint (spinal joint space) as part of a posterior cervical fusion.
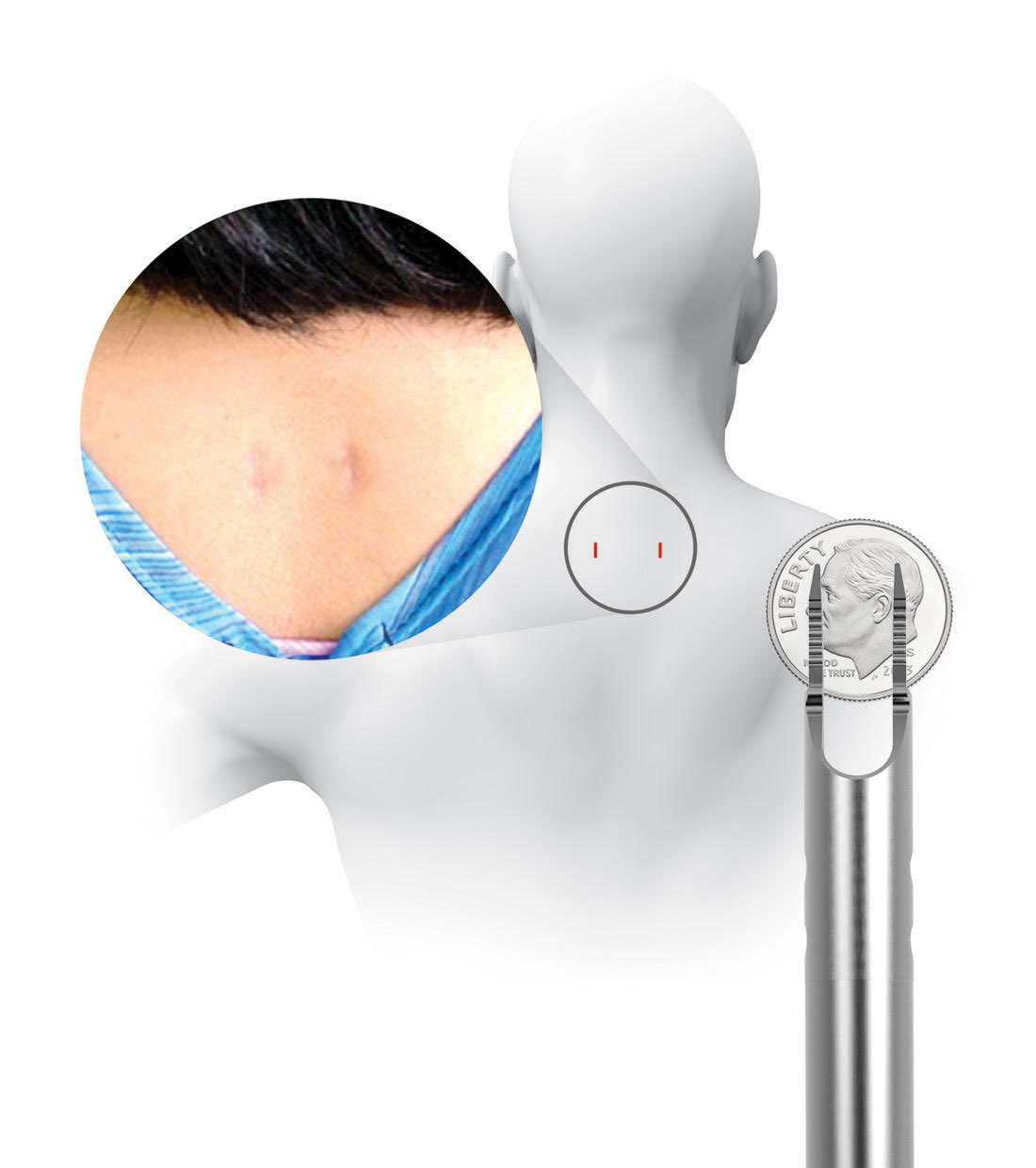
Pictured: The location of incisions and the PMT surgical instrument through which PCSS is delivered. Its diameter is less than 1 cm – smaller than a dime.
Personalized Care from Spine Specialists & Health Professionals
Receive regular, careful medical attention from a research team that includes spine surgeons and other health professionals.
Dr. Daniel Williams, MD & Dr. Alexander Lemons, MD
Dr. Daniel Williams and Dr. Alexander Lemons are the study investigators and care providers serving the greater Pinehurst, NC area.
Dr. Williams and Dr. Lemons are both board-certified & fellowship-trained orthopedic spine & neck surgeons with extensive experience in the latest spinal surgery treatments and techniques.
Pinehurst Surgical
5 First Village Drive
Pinehurst, NC 28374

Dr. Daniel Williams, MD

Dr. Alexander Lemons, MD
Contribute to Research of Spinal Therapies & Treatment
By participating in this study, you will be helping to advance the understanding of spinal surgery, which may improve care for future patients.
Study Participants May Receive Compensation
Patients who agree to participate in the study may be compensated for their time and study travel costs. Payment details will be provided by your doctor.
Request Study Brochure
By submitting this form you agree to our Terms of Use.
We respect your privacy. Privacy Policy
What clinical information is already available for PCSS?
PCSS is an investigational device. Providence Medical Technology, the company that designed PCSS, conducted a clinical study in the Philippines using a similar device that was also placed into the facet joints.
This study demonstrated:9,10
- A decrease in pain
- Lower Neck Disability Index (NDI) scores — a quality of life scale
- High fusion rate
In addition, surgeons in the U.S. have used the PCSS and similar devices in their patients with favorable outcomes and a low reported device-related complication rate of 3.4%.11
In a biomechanical study, the combination of ACDF (anterior fusion) and a PCF (posterior fusion) using PCSS significantly increased spinal stability versus ACDF alone.12
Prior to deciding whether to participate in the study, please discuss the risks and benefits of PCSS and the study with your doctor.
Official Study Name
Safety and Effectiveness of Posterior Cervical Stabilization System (PCSS) as an adjunct to posterior cervical fusion (PCF), when used in combination with ACDF in the treatment of multi-level cervical degenerative disease. (“FUSE Study”)
Learn more at www.fusestudy.com
For detailed information about this cervical fusion clinical study visit: clinicaltrials.gov
CAUTION — PCSS is an investigational device. Limited by federal law to investigational use.

About Providence Medical Technology
Our Purpose is to improve clinical outcomes for high-risk patients and prevent surgical failures of the cervical spine.
Our Mission is to establish Circumferential Cervical Fusion (CCF) as the standard of care for high-risk patients.
3875 Hopyard Rd. Suite 300
Pleasanton, CA 94588
References
1. Lu J, et al. (2000) Clin Orthop Relat Res.
2. Yu BS, et al. (2017) Nerve.
3. Shen HX, et al. (2010) Spine (Phila Pa 1976).
4. Zigler JE, et al. (2016) Spine (Phila Pa 1976).
5. Song KJ, et al. (2010) J. Bone Joint Surg. Br.
6. Dmitriev AE, et al. (2007) Spine (Phila Pa 1976).
7. Mummaneni PV, et al. (2007) Neurosurgery.
8. Harel R, et al. (2013) J Spinal Disord Tech.
9. McCormack B, et al. (2013) J Neurosurg Spine.
10. Siemionow K, et al. (2016) J Neurol Surg A Cent Eur Neurosurg.
11. Siemionow K, et al. (2017) J Craniovertebr Junction Spine.
12. Voronov LI, et al. (2016) Medical Devices (Auckl).
* Providence products were not used in these studies.
Copyright © 2021, Providence Medical Technology. All rights reserved.